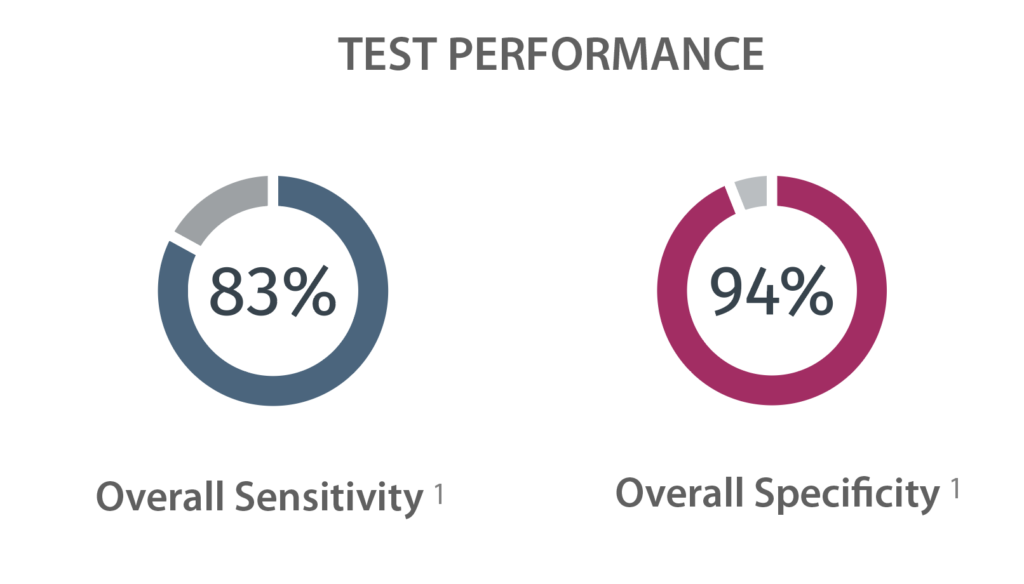
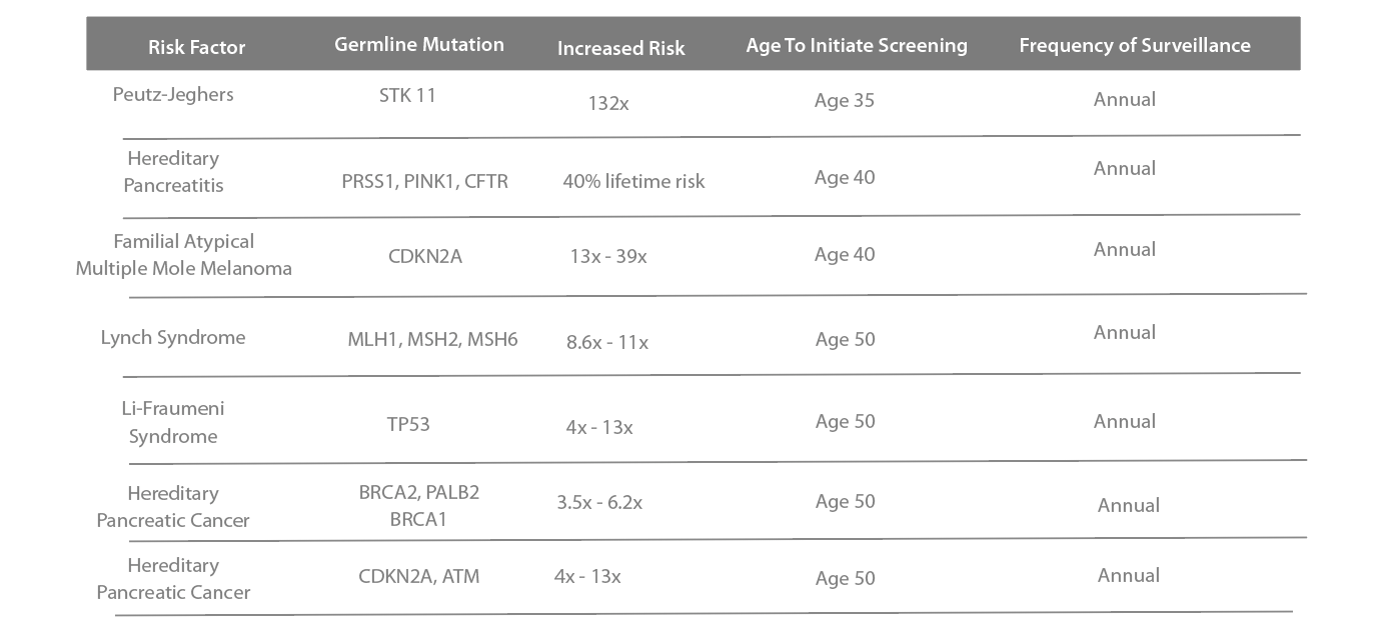
Regular screening for patients at high risk to develop pancreatic cancer can bring peace-of-mind, increased chances of survival, and a new way to combat one of the most deadly cancers affecting communities across the U.S.
The test works by looking at 59 clinically-validated DNA methylation regions that originate from cell-free tumor DNA molecules that circulate in the blood. These indicatory biochemical signatures can be detected on a per base level and are specific to pancreatic cancer.