There are new ways to treat pancreatic cancer, but they still depend
on the initiation and follow through of an early detection strategy
Improving Pancreatic Cancer Survival Rates
A 3-Step Process for Providers to Consider
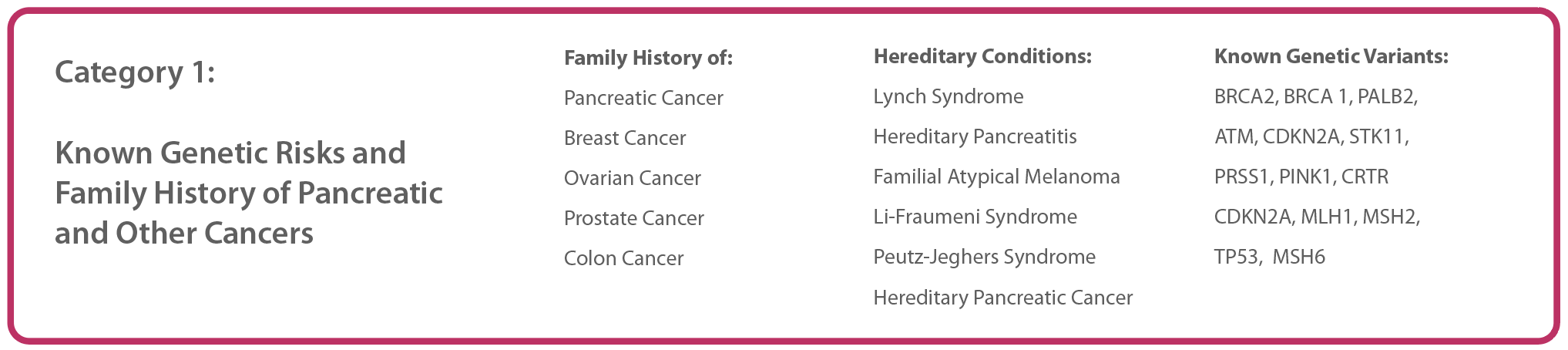
1. Identify patients who may have an elevated risk
New studies continue to expand the list of clinical conditions and family history/genetics that should be taken into account when determining whether a patient is at high risk
2. Develop a clear surveillance strategy
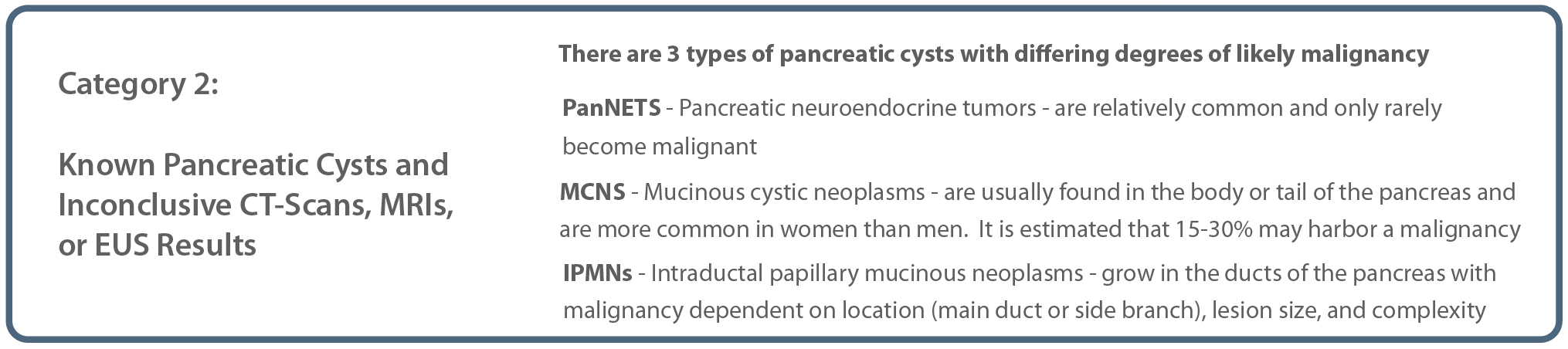
It is recommended that high risk patients begin a screening regimen starting at age 50, with shortened intervals for patients with specific genetic variants, concerning pancreatic cysts, or certain unresolved clinical conditions
3. Discuss screening options with patients and the benefits of a non-invasive approach
Adherence is key. BT-RevealTM offers an effective and convenient way to screen patients for pancreatic cancer without the risk of side effects or the high costs and scheduling difficulties associated with traditional image-based techniques. In their most recent clinical practice update, the American Gastroenterology Association highlighted the need for “biochemical tests” to complement CA19-9 and image-based techniques.1
Categories of Clinical Use

Recommendations
Following professional guidelines for screening of high risk individuals by the American Gastroenterology Association (AGA) and the American Society of Gastrointestinal Endoscopy (ASGE), the BT-RevealTM Early Pancreatic Cancer Test is generally recommended as a yearly screening test with shortened intervals for patients with specific genetic risk factors or concerning clinical conditions1, 3. While no specific recommendations have been given by professional organizations in regards to this test or other blood-based screening tests for pancreatic cancer, statements by the AGA in their most recent clinical practice guidelines highlight the clear need for a safe and non-invasive “biochemical test” to complement existing image-based approaches including CT-Scans, MRIs, and EUS.2
A long-term study published with support of the International Cancer of the Pancreas Screening Consortium (CAPS) showed that most pancreatic cancers (PDAC) discovered during patient surveillance were resectable and that 85% of those patients survived for three years or more4.
References
1 Aslanian HR, Lee JH, Canto MI. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology. 2020 Jul;159(1):358-362. doi: 10.1053/j.gastro.2020.03.088. Epub 2020 May 19. PMID: 32416142
2 Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021 Apr 1;19(4):439-457. doi: 10.6004/jnccn.2021.0017. PMID: 33845462
3 Sawhney MS, Calderwood AH, Thosani NC, Rebbeck TR, Wani S, Canto MI, Fishman DS, Golan T, Hidalgo M, Kwon RS, Riegert-Johnson DL, Sahani DV, Stoffel EM, Vollmer CM Jr, Qumseya BJ; Prepared by: ASGE STANDARDS OF PRACTICE COMMITTEE. ASGE guideline on screening for pancreatic cancer in individuals with genetic susceptibility: summary and recommendations. Gastrointest Endosc. 2022 May;95(5):817-826. doi: 10.1016/j.gie.2021.12.001. Epub 2022 Feb 16. PMID: 35183358
4 Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, Bartsch DK, Bassi C, Carrato A, Farrell J, Fishman EK, Fockens P, Gress TM, van Hooft JE, Hruban RH, Kastrinos F, Klein A, Lennon AM, Lucas A, Park W, Rustgi A, Simeone D, Stoffel E, Vasen HFA, Cahen DL, Canto MI, Bruno M; International Cancer of the Pancreas Screening (CAPS) consortium. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2020 Jan;69(1):7-17. doi: 10.1136/gutjnl-2019-319352. Epub 2019 Oct 31. Erratum in: Gut. 2020 Jun;69(6):e3. PMID: 31672839; PMCID: PMC7295005